Whole Grain Brewing Beef Cattle Journal of Animal Science
- Research
- Open Access
- Published:
Feed nutritional value of brewers' spent grain residue resulting from protease aided poly peptide removal
Periodical of Animal Scientific discipline and Biotechnology volume 10, Article number:78 (2019) Cite this commodity
Abstract
Background
This study was conducted to evaluate the feed nutritional value of brewers' spent grain (BSG) residue resulting from protease aided protein removal. The nutritional value was measured as food content, gas production, nutrient digestibility and fermentation characteristics in batch culture.
Results
Protein extraction process decreased content of crude poly peptide but concentrated the neutral detergent fibre (NDF) and ferulic acid in BSG residue. The changes in the chemic limerick of BSG residue varied with enzyme and enzyme dosage. Digestibility of dry thing (DMD) and NDF of residual differed among proteases. Increasing alcalase dosage linearly decreased DMD, whereas, the DMD linearly increased equally everlase or flavourzyme dosage increased. Compared with BSG, the DMD, gas product and fermentation acid concentration of BSG residues were lower, whereas NDF digestibility was college.
Conclusions
The essentially increased NDF content and improved in vitro NDF digestibility due to protease hydrolysis suggest that BSG residual can exist potentially exploited as a viable fibre source for ruminant feeding.
Background
Brewers' spent grain (BSG) is the major by-product of beer industry, representing ~ 85% of the total by-products generated [1]. Beer is the fifth nigh consumed beverage in the world, resulting an average annual global production of 39 million tonnes of BSG [1]. Brewing removes the soluble part of the grain, thus concentrating insoluble textile in BSG. This includes 15–26% poly peptide and 35–60% fibre on dry basis [2]. Despite the fact that BSG contains pregnant levels of valuable poly peptide and fibre, its main awarding is animal feeding, particularly cattle. All the same, there is an increasing research involvement in protease hydrolysis of BSG to produce bioactive peptides which could be used for nutrient and nutraceutical applications [3,4,v]. However, after extraction of peptides, the insoluble balance containing carbohydrate has been treated as waste without any high-value awarding. Faulds et al. [vi] treated BSG with unlike proteases and institute that proteases have the ability to solubilise BSG carbohydrate components in add-on to protein. It has been suggested that the carbohydrate jump with protein can be released upon protease hydrolysis [6]. Thus, it is hypothesised that protease hydrolysis of BSG may increase the digestibility of BSG rest, and thus increase its feed value. Information technology is besides expected that protease treated BSG may have high fibre content in residuum, due to the partial removal of protein.
In improver, it was found that BSG is a adept source of phenolic antioxidants including ferulic acid and p-coumaric acrid [1]. Since most of the phenolic compounds are trapped in the cell walls of cereals, those are not solubilized in brewing, thus get concentrated in BSG [1]. Faulds et al. [6] establish that ferulic acid was released upon protease hydrolysis of BSG. Ferulic acid can adversely affect the fibre digestibility [vii]. Therefore, the objectives of this study were to prepare BSG residue by protease hydrolysis with different enzymes at different dosages, and evaluate their food digestibility, in vitro gas production, and fermentation characteristics using batch culture technique.
Methods
Preparation of BSG residuum
Three BSG samples from different batches were obtained from a local brewery, and stored at − twenty °C until used. Alcalase (protease from Bacillus licheniformis, ≥ 2.4 U/g), everlase (protease from Bacillus sp., ≥ sixteen U/g), flavourzyme (protease from Aspergillus oryzae, ≥ 500 U/g) and viscozyme (carbohydrase from Aspergillus sp., 100 Fungal Beta-Glucanase U/g) were purchased from Sigma (St. Louis, USA). Sample was beginning hydrolyzed with viscozyme to solubilize carbohydrates and and then with proteases to release carbohydrates spring to proteins based on the method developed by Xia et al. [eight]. In detail, 100 g of BSG was milled to 0.v mm and solubilized in 500 mL of h2o to prepare twenty% (west/5) solution. Then information technology was kept stirring at 350 r/min for 30 min followed by adjusting to pH v and 50 °C which are the optimum weather for viscozyme. Sample was hydrolyzed with 2% (westward/westward) viscozyme for 1 h, stirring at 350 r/min. Aforementioned procedure was followed to prepare 9 samples for alcalase, everlase and flavourzyme hydrolysis at 3 dissimilar enzyme to protein ratios (w/w): one:100 (Low), v:100 (Med), x:100 (MedHigh), 15:100 (Loftier). Before adding proteases, pH and temperature were adjusted to the optimum of each enzyme, which were pH 8 and 55 °C for alcalase, pH 9.5 and 55 °C for everlase, and pH 6.6 and 55 °C for flavourzyme. The pH and temperature were monitored throughout hydrolysis. After lx min, samples were heated in a water bath for 20 min at 80 °C to inactivate enzymes. The samples were then kept stirring at 350 r/min for 30 min at pH 10.five to solubilize proteins. The solubilized proteins were separated from BSG balance by centrifuging at 8000×1000 for 15 min at twenty °C. The precipitate containing insoluble residuum was oven dried at 60 °C for 24 h. The oven-dried BSG residues were stored at − 20 °C until in vitro evaluation [8].
Experimental design, substrate and inoculum
The experiment was a consummate randomized design with a factorial arrangement of treatments; 3 proteases (alcalase, everlase and flavourzyme) × 4 dosages of each enzyme + BSG (original) with 3 replications (bottles) per handling combination. The proteases and their dosages were selected based on the preliminary results. The substrates were the BSG residues generated as described higher up during protein extraction process. The civilisation was repeated in two runs (batches). Doses of enzyme were expressed as ratio of enzyme to substrate protein (w/w), i:100, 5:100, 10:100, 15:100, respectively, for Low, Med, MedHigh and Loftier. Rumen inoculum was obtained from three ruminally fistulated Angus beef heifers (650 kg of body weight) fed a diet consisting of threescore% barley silage, 30% barley straw and 10% protein, vitamin and mineral supplement (DM ground). A high-forage diet was fed to rumen inoculum donor heifers because the substrate was a loftier-fibre feed. Nutrient composition of the nutrition was 12.one% rough protein (CP), 50.2% neutral detergent fibre (NDF) and 14.i% starch (DM ground). A total mixed ration was prepared daily using a feed mixer (Data Ranger, American Calan Inc., Northwood, NH, Us) and offered twice daily (morning time and afternoon). The animals were cared according to the guidelines of the Canadian Council on Animal Intendance [9].
Batch civilisation procedures
The incubations were performed in triplicate of each treatment combination using drinking glass bottles (100 mL) with rubber stoppers and aluminum caps. Approximately 0.5 yard (DM footing) BSG or BSG remainder was weighed into acetone-washed and pre-weighed filter bags (F57; porosity: 25 μm, Ankom Technology, Macedon, NY, U.s.) and hot sealed. Sealed bags were placed in 100 mL fermentation bottle gently. Ruminal contents were collected ii h before morning feeding from different locations within the rumen and squeezed through iv layers of cheesecloth. The squeezed ruminal fluids from 3 animals were mixed and the pH was measured immediately using a pH meter (B20PI, SymHony Benchtop Meters; VWR Edmonton, AB, Canada). The pH of ruminal fluid during this study ranged from six.5 to half-dozen.eight for the entire experiment. The ruminal fluid was stored in an air tight pre-heated (39 °C) container, transferred to the laboratory inside 10 min, and re-strained through iv layers cheesecloth into a container at 39 °C water bath with bubbling of COii. Forty-five mL McDougall'southward buffer and 15 mL ruminal fluid were added into the fermentation bottle, oxygen free CO2 was used to flushed and 14-mm butyl prophylactic stopper and aluminum seal were used to seal the bottle. Sealed bottles were placed on an oscillating shaker (125 r/min of oscillation speed) in a 39 °C incubator for 24 h incubation. Triplicates were used for every sample in each run. Two runs were carried out within 2 weeks. Additionally, three blank controls with empty Ankom bags simply were used to correct gas production in each run.
Gas pressure was recorded at 3, 6, 9, 12 and 24 h of incubation using a pressure transducer (model PX4200-015GI, Omega Engineering, Inc., Laval, QC, Canada) with a 23 approximate needle (0.vi mm) through rubber stoppers. Then gas was vented by leaving the needle in place and removing the transducer. Pressure values, corrected for the gas released from the blanks, were used to generate volume estimates using the equation of Mauricio et al. [10]: GPt (mL) = 0.18 + 3.697Pt + 0.0824Pt 2, where GPt is the gas production volume at time 't' (h), Pt is the gas pressure measured at time 't' (h).
Kinetics of gas product was generated by plumbing fixtures gas production data to an exponential model [11] as: y = GV × (i – due east– c × (t – lag)), where 'y' is the cumulative volume of gas produced at time 't' (h), 'GV' is the asymptotic gas volume, 'c' is the gas production charge per unit and 'lag' is the time (h) between inoculation and commencement of gas production.
After 24 h of incubation, fermentation bottles were placed into water ice to finish the fermentation. Fermentation media pH was measured immediately using a pH meter (B20PI, SymHony Benchtop Meters; VWR Edmonton, AB, Canada). Subsample of five mL fermentation liquid was mixed with 1 mL 25% (west/v) HPOthree for volatile fatty acrid (VFA) analysis and another 5 mL fermentation media was mixed with 1 mL 1% (w/5) HiiThen4 solution used for NH3-N analysis. The bags were removed from the bottles, washed manually under tap water and oven dried at 55 °C for 48 h to determine the DM digestibility (DMD). The DMD was calculated past the weight loss of the substrate.
Chemical analyses
Samples of BSG and BSG residue before and later on incubation were analyzed for DM (903.15), acrid detergent fibre (ADF; 973.18) and ash (942.05) according to the standard methods of AOAC [12]. The OM content was calculated as 100 minus ash content. The total North content in the BSG and BSG balance was analyzed using wink combustion and thermal conductivity detection technique (model 1500, Carlo Erba Instruments, Milan, Italia) and CP was calculated as Northward × 6.25. The NDF content in the BSG and BSG residue before and afterwards incubation was determined according to Larbi et al. [thirteen] using heat-stable α-amylase (Termamyl 120 50, Novo NordiskBiochem, Franklinton, NC, USA) with sodium sulfite. The NDF and ADF contents were expressed with ash inclusion. Hemicellulose was calculated equally the difference between NDF and ADF. Not-fibre carbohydrate (NFC) was calculated every bit OM content minus the sum of NDF, ether extract and CP contents. The concentration of VFA in fermentation media was analysed using a gas chromatograph (model 5890, Hewlett-Packard Lab, Palo Alto, CA, USA) equipped with a capillary cavalcade (xxx yard × 0.32 mm i.d., one-μm stage thickness, Zebron ZB-FAAP, Phenomenex, Torrance, CA, Usa) and flame ionization detection. The concentration of NHthree-N in fermentation media was determined equally described by Rhine et al. [14]. Total ferulic acrid content in BSG and BSG residue was analysed as described by Cao et al. [15] using HPLC with a Symmetry opposite phase C-xviii column (250 mm × 4.6 mm i.d., 5-μm stage thickness; Waters, Milford, MA, USA).
Statistical analyses
Data were analysed using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC, USA) with model including enzymes, enzyme dosages and the interaction as stock-still effects, replicates of BSG and BSG residual extracted by aforementioned enzyme and different runs were considered as random effects. The effect of increasing enzyme dosages was examined through linear and quadratic orthogonal contrasts using the Contrast argument of SAS. Contrasts were generated to compare the boilerplate of four enzyme dosages and original BSG. The PDIFF choice adjusted by the Turkey method was included in the LSMEANS statement to account for multiple comparisons among treatments. Differences between treatments were declared significant at P ≤ 0.05. Trends were discussed at 0.05 <P ≤ 0.10. The Pearson correlation coefficient was analysed with the CORR process of SAS (SAS Inst. Inc., Cary, NC, USA).
Results & discussion
Effect of protease hydrolysis on chemical composition of BSG residue
The BSG is considered as a lignocellulosic textile rich in protein and fibre, which may vary substantially, ranging from 15% to 35% and 43% to 73%, respectively [16,17,18]. The considerable variation in the chemical composition of BSG amid studies could be due to differences in barley multifariousness, harvest fourth dimension, malting and mashing conditions, and the quality and type of additives added in the brewing process [19]. The CP (25.6%) and NDF (46.vi%) contents of the BSG used in our protein extraction is within the range reported in the literature.
There were interactions betwixt enzyme and enzyme dosages on the contents of OM, NDF, ADF and CP of BSG residues; although statistically significant, the differences in the OM, NDF and ADF contents of BSG rest among enzymes were quantitatively small (Table i). Withal, the CP content of the residue treated with flavourzyme was greater (P < 0.01) than the alcalase or everlase-treated residues regardless of the enzyme dosages used. In addition, the CP content of the remainder treated with everlase was also greater (P < 0.05) than acalase-treated residue at Depression or Med dose. Celus et al. [20] used different enzymes to generate BSG protein hydrolysates and found that both alcalase and flavourzyme improved the poly peptide solubility, whereas the alcalase seemed to have greater activity than flavourzyme [20]. Therefore, this is a probable explanation for greater protein content left over in the BSG residuum treated with flavourzyme than with alcalase. Though the employ of everlase to hydrolyse BSG protein was seldom reported, the changes of CP content in BSG remainder in present study illustrated that alcalase may have greater activity in protein solubility than everlase at Depression or Med dose but both enzymes had the similar activity at MedHigh and High dose. The minor quantitative differences in the content of OM and NDF with increasing enzyme dose in present results are consistent with previous studies that showed oft picayune effect of protease dose-response on BSG solubility [21]. Treimo et al. [22] reported that a reduction of the peptidase dosages from 20 to five μL/chiliad DM reduced DM and poly peptide yields of BSG residue simply from 38% to 36% and from 72% to 67%, respectively. The marginal gain on BSG DM and poly peptide solubility obtained by considerable increases in alcalase dosages from ane.2 to 20 μL was also limited in the written report of Treimo et al. [23]. These results suggest that the protease dosage attributed limited effects on solubilizing DM and protein of BSG.
Ferulic acid content in BSG rest differed among enzymes at Med and MedHigh enzyme dose, and linearly (P < 0.02) increased with increasing alcalase dose, whereas information technology was non afflicted with increasing the dosages of everlase or flavourzyme (Tabular array 1). Total ferulic acid content increased (P < 0.01) in BSG residue treated with alcalase or everlase compared to the BSG, merely no difference was observed between BSG and BSG residuum treated with flavourzyme. Ferulic acid is the phenolic acid mainly existed in plant cell walls, usually links with arabinoxylans, and provides more than enzyme resistance in fibre digestion [7]. Ikram et al. [two] reported 0.34% of ferulic acid in BSG, which is similar to the value of 0.30% observed in the nowadays report. Since ferulic acid is mainly associated with hemicellulose, the greater content of hemicellulose in BSG residue resulted with greater ferulic acrid content in present written report was expected. Notwithstanding, the hemicellulose content increased by 60% in BSG residuum vs. BSG (average 41.4% vs. 25.8%), while the ferulic acid content increased only by 22% in the BSG residue vs. BSG (average 0.36% vs. 0.30%). Previous studies recovered that alcalase had the activeness to release ferulic acid from BSG [24, 25]. Flavourzyme was also reported to accept high activity in hydrolysis of the ferulate [26]. It suggests that a portion of ferulic acrid in BSG was loss equally soluble ferulic acid due to protease hydrolysis.
During protease hydrolysis of BSG, the NDF content was concentrated, while the CP and NFC content considerably reduced in the BSG remainder compared with BSG without obviously changing the OM content (Tabular array 1). The NFC in BSG could exist mainly the starch and some oligosaccharide similar glucose or maltose, which should be readily digestible. The dramatically decreased content of NFC in the balance compared with BSG suggested that the proteases might have carbohydrase activity, particularly for flavourzyme, in which the NFC in the residue was mostly gone. Faulds et al. [6] found considerable reduction of oligomers in protease-treated BSG. Xiros and Christakopoulos [27] also reported that alcalase combined with other enzymes, hydrolysed upward to 36% BSG into soluble fraction and monosaccharides.
Effect of protease hydrolysis on digestibility of BSG and BSG rest
In that location was consequent interaction between enzyme and enzyme dosage on the digestibility of DM, NDF and ADF (Fig. ane). The DMD was greatest in the remainder treated with alcalase, intermediate with everlase and lowest with flavourzyme at Low dose (P < 0.01), whereas it was greatest with everlase, intermediate with alcalase and lowest with flavourzyme at MedHigh dose (P < 0.01). With increasing alcalase doses, the DMD linearly (P < 0.01) decreased, whereas it linearly (P < 0.02) increased as everlase dose increased. The DMD of BSG residuum was lower (P < 0.01) than that of BSG (40.iv% vs. 45.6%). Digestibility of NDF was greater (P < 0.02) in the balance treated with alcalase than flavourzyme at Depression dose; it was lower (P < 0.02) with alcalase than everlase or flavourzyme at Med dose; and it was greater (P < 0.05) with everlase than flavourzyme at MedHigh dose with no difference at High dose. The ADF digestibility was different amidst enzymes but at Depression dose. With increasing alcalase doses, the digestibility of NDF and ADF quadratically (P < 0.01) decreased, whereas no response of everlase or flavourzyme dose was observed on the digestibility of NDF and ADF. The digestibility of NDF was greater (P < 0.01) merely that of ADF was lower (P < 0.01) for BSG residual than for BSG. The DMD is the boilerplate digestibility of individual nutrient including primarily NFC (starch, sugars), fibre and CP. In the present study, the lower DMD of BSG remainder than that of BSG was mainly due to decreased NFC (residue vs. BSG; average 5.v% vs. eighteen.ane%) and CP content (rest vs. BSG; average xi.5% vs. 25.vi%) in the residue. Although the NDF digestibility of residue was greater, it was offset by the lower ADF digestibility compared with that of BSG. Similarly, the overall lowest DMD of flavourzyme treated remainder was consistent with the lowest NFC content of the residue. The NFC is readily digestible, CP is the next and fibre is everyman digestible nutrient in the digestive tract of ruminants [28]. This was further supported by Pearson correlation analysis that the DMD was positively correlated with NFC content (r = 0.75; P < 0.01; Tabular array ii), but it moderately and negatively related to the NDF content (r = − 0.49, P < 0.01).
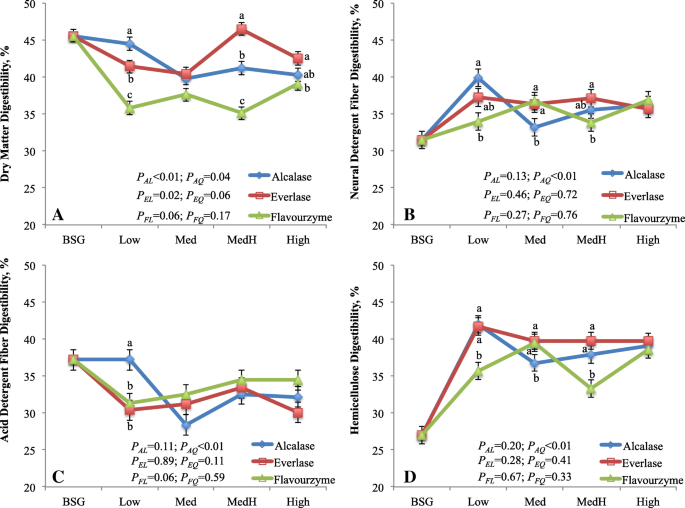
In vitro digestibility of brewers' spent grain and residues treated by proteases varying with dosages. a-c Ways with different letters with the aforementioned dose of enzyme are different (P < 0.05). BSG, brewers' spent grain; AL, EL, FL are linear and AQ, EQ, FQ are quadratic effects, respectively, for alcalase, everlase and flavouezyme dose (enzyme:protein, due west/w; 1:100, five:100, 10:100 and xv:100, respectively for Low, Med, MedH and High). All enzyme × enzyme dosage and the contrasts betwixt the average of 4 doses of each enzyme and BSG were meaning (P < 0.01). The enzyme effects were P < 0.01, P < 0.38, P < 0.xviii and P < 0.01 for Figure a, b, c and d, respectively
The method that adult for NDF analysis in fauna nutrition is to determine the fibre for its biological nature rather than for chemical nature, thus the NDF is mainly composed of hemicellulose, cellulose and lignin. The hemicellulose has greater digestibility than cellulose, and lignin is normally indigestible in the digestive tract of livestock animals [29]. Therefore, the improved NDF digestibility of the residue versus BSG was particularly due to greater hemicellulose digestibility in residuum (39%) than in BSG (27%) likewise as slightly greater hemicellulose proportion in the residue than in BSG (58 vs. 55% of NDF). The NDF digestibility is highly correlated with the digestibility of hemicellulose (r = 0.87, P < 0.01; Table two). The improved hemicellulose digestibility was probable resulted from partly release of ferulic acrid from residue during protease extraction. In BSG, arabinoxylans is one of the principal components of hemicellulose and contain large amount of ferulic acrid [30]. Ferulic acid can esterify to arabinose, enlarge the molecular weight of arabinoxylans, and adversely bear upon the digestibility and solubility of arabinoxylans [7, 31]. Still, the reduction in ADF digestibility of the residual compared with BSG is not clear in present study. We speculate that the residue containing low NFC might decrease bacterial colonization on the residue, which is a critical step for the fibre digestion in the rumen [32].
Interaction of enzyme with enzyme dose on gas production kinetics was noticed, which is consistent with the digestibility results (Fig. ii). The asymptotic gas book was lower (P < 0.04) at Low dose but it was greater (P < 0.05) at High dose with flavourzyme-treated residues compared to alcalase or everlase-treated residues. Overall, gas production charge per unit was greatest (P < 0.01) in residue treated with everlase, intermediate with alcalase and lowest with flavourzyme. Increasing alcalsae dose linearly (P < 0.01) decreased asymptotic gas volume and gas production rate, while the gas production charge per unit was quadratically (P < 0.04) changed every bit everlase or flavourzyme doses increased. The asymptotic gas volume did not differ betwixt residue (93.one) and BSG (94.6 mL/thousand DM), whereas the gas production rate was lower (P < 0.01) for residue (four.30%/h) than BSG (5.38%/h). In gas product system, the asymptotic gas book and gas production rate reverberate the extent and charge per unit of the substrate fermentation, respectively, and the gas product in vitro is highly correlated to substrate digestion [33]. The linearly decreased gas production was consistent with the linear reduction of DMD with increasing alcalase doses. Notwithstanding, contrary to the DMD, the asymptotic gas volume of everlase or flavourzyme-treated residues did not show the enzyme dose response. This may be explained by differences in other fermentation products because the truly digested substrates in batch culture are divided among VFA, gas, and microbial biomass. The gas production charge per unit is highly correlated with the NFC content of the substrates [34], therefore, the similar responses to enzyme and enzyme dose between NFC content and gas production rate were observed.
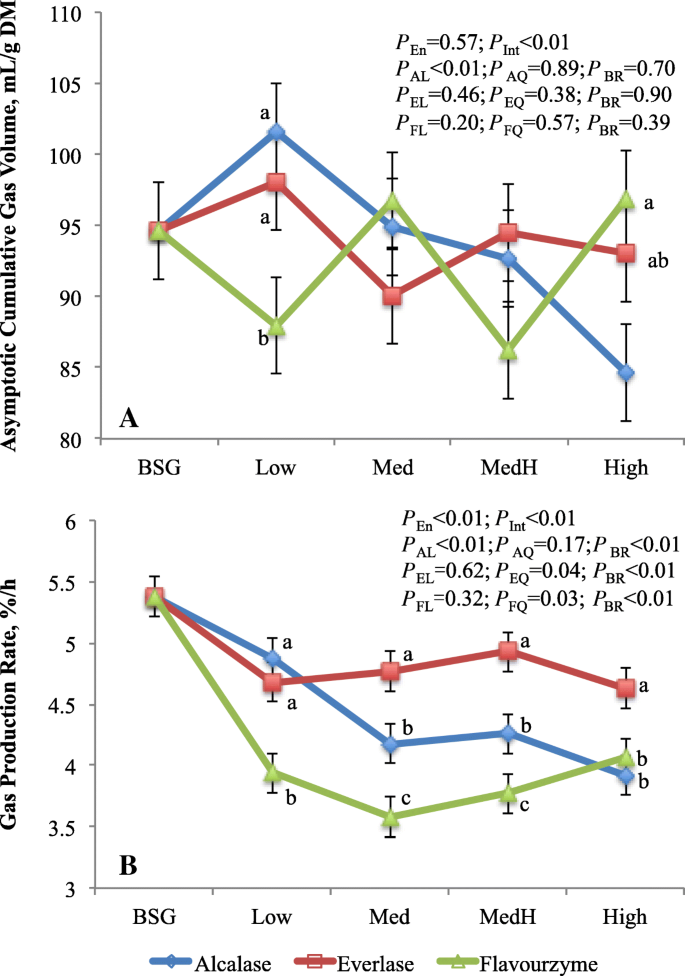
Asymptotic gas volume (mL/g DM; a) and gas production rate (%/h; b) of brewers' spent grain and residues treated by proteases varying with dosages. a-cMeans with different messages in the aforementioned dose are different (P < 0.05). BSG, brewers' spent grain; P en, enzyme effects; P Int, interaction between enzyme and enzyme dosage; P AL, P EL, P FL are linear and P AQ, P EQ, P FQ are quadratic effects of, respectively, alcalase, everlase and flavouezyme dose (enzyme:protein, w/w; 1:100, 5:100, ten:100 and xv:100, respectively for Depression, Med, MedH and Loftier); P BR, dissimilarity between average of iv doses of each enzyme and BSG
Effect of protease hydrolysis on fermentation patterns of BSG and BSG rest
The enzyme and enzyme dose interaction was noticed on VFA concentration, molar proportion of acetate, propionate and ratio of acetate to propionate (A:P; Table 3). The VFA concentration was greater (P < 0.05) in residue treated with alcalase than with flavourzyme at Low dose, which agreed with the DMD response. The VFA concentration had the like enzyme dose response as to that observed for the DMD. In add-on, the lower (P < 0.01) VFA concentration for the remainder than BSG is also in accordance with lower DMD of the residue. The differences in molar proportion of individual VFA were quantitatively modest among enzymes even though they were statistically significant. The greater proportion of acetate with remainder than with BSG might be explained by the greater NDF digestibility of the residual. The A:P in residue treated with flavourzyme was greater (P < 0.05) than with alcalase at Depression dose and than with everlase at MedHigh dose, and it quadratically (P < 0.01) increased with increasing alcalase or flavourzyme doses. The A:P was also greater (P < 0.01) with flavourzyme-treated residue than BSG. Overall, the A:P of BSG residue appeared to be depression because its loftier NDF content (> 70% of DM). The low A:P could be due to high hemicellulose content along with its high digestibility in the residue. Spud et al. [35] reported that the fermentation of hemicellulose past ruminal microbes resulted in a greater reduced A:P than with the fermentation of cellulose. The depression A:P indicates relatively greater propionate proportion, and thus greater fermentation efficiency [36]. When propionate is produced, the carbon and hydrogen from glucose are still present in VFA, whereas when acetate is produced, the office of carbon and hydrogen from glucose are produced as CO2 and marsh gas. Therefore, lack of difference in A:P between BSG and remainder treated with alcalase and everlase suggested the fermentation efficiency of the balance was not reduced.
The concentration of NH3-Due north in fermentation media was not afflicted by the treatments except that the concentration of NH3-N was slightly greater with alcalase (23.9 mmol/L; P < 0.09) and everlase (24.i mmol/Fifty; P < 0.05) treated residue than BSG (22.vi mmol/L; Tabular array 3). The NH3-N concentration from this study was similar to the previous written report in batch culture incubated loftier-grain substrate (xc% barley DM) [34]. Rumen NH3-N concentration is dynamic residuum betwixt product from proteolysis and utilization for microbial poly peptide product. Carbohydrate availability strongly influences the utilization of NHiii-N by rumen microbes. Benchaar et al. [37] observed lower ruminal NH3-Due north concentration when the starch supply increased in the diet. Therefore, the lower NH3-N concentration with BSG may be explained past the greater NFC content of BSG, which might increment the use of NHiii-Northward past microbes. However, the lack of the NH3-N concentration response to enzyme or enzyme dose was somehow not expected. For case, the college NH3-N concentration could be higher for flavourzyme-treated residue because of its higher CP contents and lower NFC content. It speculates that protein degradability of flavourzyme residue may be low.
Conclusions
In summary, the protease hydrolysis increased the fibre and ferulic acid content of the BSG residue, but decreased NFC and CP content. Although the DMD and full VFA concentration decreased, higher NDF digestibility was observed for the rest compared with BSG. The alcalase and everlase overall demonstrated amend hydrolysis efficiency on poly peptide removal and generated greater DMD in the residue when compared to flavourzyme. Increasing dosages of alcalase and everlase also increased fermentability of the rest. These results indicated that the overall feed value of the BSG residue may be slightly lower than BSG, it is a feasible fibre source in ruminant feeds. In addition, the college ferulic acid content of the residue is warranted further study for its antioxidant activeness.
Availability of data and materials
The information analyzed during the current study are bachelor from the corresponding writer on reasonable asking.
Abbreviations
- A:
-
P ratio of acetate to propionate
- ADF:
-
Acid detergent fibre
- BSG:
-
Brewers' spent grain
- CP:
-
Crude poly peptide
- DM:
-
Dry matter
- DMD:
-
DM disappearance
- Med:
-
Medium
- MedHigh:
-
Medium loftier
- NDF:
-
Neutral detergent fibre
- NFC:
-
Non-fibre carbohydrate
- VFA:
-
Volatile fatty acid
References
-
Mussatto SI. Brewer's spent grain: a valuable feedstock for industrial applications. J Sci Food Agric. 2014;94:1264–75.
-
Ikram S, Huang Fifty, Zhang H, Wang J, Yin G. Composition and nutrient value proposition of brewers spent grain. J Nutrient Sci. 2017;82:2232–42.
-
Connolly A, O'keeffe MB, Nongonierma AB, Piggott CO, FitzGerald RJ. Isolation of peptides from a novel brewers spent grain protein isolate with potential to modulate glycaemic response. Int J Food Sci Technol. 2017;52:146–53.
-
Lynch KM, Steffen EJ, Arendt EK. Brewers' spent grain: a review with an emphasis on food and health. J Inst Brew. 2016;122:553–68.
-
Vieira EF, da Silva DD, Carmo H, Ferreira IM. Protective power against oxidative stress of brewers' spent grain protein hydrolysates. Nutrient Chem. 2017;228:602–9.
-
Faulds CB, Collins S, Robertson JA, Treimo J, Eijsink VGH, Hinz SWA, et al. Protease-induced solubilisation of carbohydrates from brewers' spent grain. J Cereal Sci. 2009;50:332–6.
-
Appeldoorn MM, Waard PD, Kabel MA, Gruppen H, Schols HA. Enzyme resistant feruloylated xylooligomer analogues from thermochemically treated corn fibre contain large side chains, ethyl glycosides and novel sites of acetylation. Carbohydr Res. 2013;381:33–42.
-
Xia YC, Bamdad F, Gänzle M, Chen 50. Fractionation and label of antioxidant peptides derived from barley glutelin past enzymatic hydrolysis. Food Chem. 2012;134:1509–18.
-
Canadian Council on Fauna Care. Guide to the intendance and utilize of subcontract animals in research teaching and testing. E.D. Olfert, B.M. Cross, and A.A. McWilliam, ed. Ottawa: Tin can Counc Anim Care; 2009.
-
Mauricio RM, Mould FL, Dhanoa MS, Owen E, Channa KS, Theodorou MK. A semi-automatic in vitro gas production technique for ruminant feedstuff evaluation. Anim Feed Sci Technol. 1999;79:321–30.
-
Ørskov ER, McDonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci. 1979;92:499–503.
-
AOAC, editor. Official methods of analysis. 18th ed. Gaithersburg: AOAC Int; 2005.
-
Larbi A, Smith JW, Adekunle IO, Kurdi IO. Studies on multipurpose provender copse and shrubs in West Africa: variation in determinants of forage quality in Albizia and Paraserianthes species. Agrofor Syst. 1996;33:29–39.
-
Rhine ED, Mulvaney RL, Pratt EJ, Sims GK. Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Sci Soc Am J. 1998;62:473–fourscore.
-
Cao BB, Jin X, Yang HJ, Li SL, Jiang LS. Microbial release of ferulic and p-coumaric acids from forages and their digestibility in lactating cows fed full mixed rations with dissimilar fodder combinations. J Sci Nutrient Agric. 2016;96:650–five.
-
McCarthy AL, O'Callaghan YC, Piggott CO, FitzGerald RJ, O'Brien NM. Brewers' spent grain; bioactivity of phenolic component, its role in brute diet and potential for incorporation in functional foods: a review. Proc Nutr Soc. 2013;72:117–25.
-
Mussatto SI, Dragone 1000, Roberto IC. Brewers' spent grain: generation, characteristics and potential applications. J Cereal Sci. 2006;43:ane–fourteen.
-
Vieira E, Rocha MAM, Coelho E, Pinho O, Saraiva JA, Ferreira IM, et al. Valuation of brewer'southward spent grain using a fully recyclable integrated process for extraction of proteins and arabinoxylans. Ind Crops Prod. 2014;52:136–43.
-
Santos M, Jimenez JJ, Bartolome B, Gomezcordoves C, Del Nosal MJ. Variability of brewer'south spent grain within a brewery. Nutrient Chem. 2003;80:17–21.
-
Celus I, Brijs K, Delcour JA. Enzymatic hydrolysis of brewers' spent grain proteins and technofunctional properties of the resulting hydrolysates. J Agric Nutrient Chem. 2007;55:8703.
-
Faulds CB, Robertson JA, Waldron KW. Issue of pH on the solubilization of brewers' spent grain by microbial carbohydrases and proteases. J Agric Food Chem. 2008;56:7038–43.
-
Treimo J, Westereng B, Horn SJ, Forssell P, Robertson JA, Faulds CB, et al. Enzymatic solubilization of brewers' spent grain by combined activity of carbohydrases and peptidases. J Agric Food Chem. 2009;57:3316–24.
-
Treimo J, Aspmo SI, Eijsink VG, Horn SJ. Enzymatic solubilization of proteins in brewer'southward spent grain. J Agric Food Chem. 2008;56:5359–65.
-
Charilaos Ten, Maria M, Evangelos T, Paul C. Factors affecting ferulic acrid release from Brewer'southward spent grain by Fusarium oxysporum enzymatic system. Bioresour Technol. 2009;100:5917–21.
-
Robertson JA, Luis CMA, Collins SRA, Faulds CB, Waldron KW. Enzymatic and chemical treatment limits on the controlled solubilization of brewers' spent grain. J Agric Food Chem. 2011;59:11019–25.
-
Couto J, Karboune S, Mathew R. Regioselective synthesis of feruloylated glycosides using the feruloyl esterases expressed in selected commercial multi-enzymatic preparations as biocatalysts. Biocatalysis. 2010;28:235–44.
-
Xiros C, Christakopoulos P. Biotechnological potential of brewers spent grain and its recent applications. Waste product Biomass Valoriz. 2012;3:213–32.
-
National Academies of Sciences, Technology, and Medicine (NASEM). Nutrient requirements of beefiness cattle. eighth ed. Washington DC: Natl. Acad. Press; 2016.
-
Keys JE Jr, Van Soest PJ, Young EP. Comparative study of the digestibility of forage cellulose and hemicellulose in ruminants and nonruminants. J Anim Sci. 1969;29:11.
-
Mandalari K, Faulds CB, Sancho AI, Saija A, Bisignano Yard, Locurto R, et al. Fractionation and characterisation of arabinoxylans from brewers spent grain and wheat bran. J Cereal Sci. 2005;42:205–12.
-
Dervilly-Pinel G, Rimsten 50, Saulnier L, Andersson R, Åman P. H2o-extractable Arabinoxylan from pearled flours of wheat, barley, Rye and triticale. Evidence for the presence of Ferulic acrid dimers and their interest in gel formation. J Cereal Sci. 2001;34:207–14.
-
Cheng KJ, McAllister TA, Selinger LB, Yanke LJ, Bae Hd, Forsberg CW, et al. In Biotechnology in the feedlot, Proceedings-American Lodge of Animal Scientific discipline Western Section. New Las Cruces: Mexico State University; 1995. pp 600–605.
-
Blümmel Grand, Ørskov ER. Comparing of in vitro gas product and nylon pocketbook degradability of roughages in predicting feed intake in cattle. Anim Feed Sci Technol. 1993;40:109–19.
-
Jiao PX, Liu FZ, Beauchemin KA, Yang WZ. Impact of strain and dose of lactic acrid bacteria on in vitro ruminal fermentation with varying media pH levels and feed substrates. Anim Feed Sci Technol. 2017;224:ane–13.
-
Murphy MR, Baldwin RL, Koong LJ. Interpretation of stoichiometric parameters for rumen fermentation of roughage and concentrate diets. J Anim Sci. 1982;55:411–21.
-
Cheeke PR, Dierenfeld ES. Comparative animal nutrition and metabolism. Cambridge: Cambridge University Press; 2010.
-
Benchaar C, Hassanat F, Gervais R, Chouinard PY, Petit HV, Massé DI. Methane production, digestion, ruminal fermentation, nitrogen balance, and milk production of cows fed corn silage-or barley silage-based diets. J Dairy Sci. 2014;97:961–74.
Acknowledgements
The authors give thanks the Lethbridge Research and Evolution Centre Metabolism barn staff for their care and management of the animals and Alastair Furtado and Darrell Vedres for their technical assistance.
Funding
This inquiry was funded by AAFC Growing forward program (GF2#1542).
Writer information
Authors and Affiliations
Contributions
WZY and LYC designed the study and revised manuscript, RA performed BSG residue development and revised manuscript; TR, XS and JGL helped on samples collection and analysis. YZS performed all experiment, analysed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All the procedures for the treatment and care of experimental cattle were approved by the Creature Care and Utilise Committee of Lethbridge Research and Development Centre and followed the guidelines for the Canadian Council on Beast Care (2009).
Consent for publication
Non applicable.
Competing interests
The authors declare that they accept no competing interests.
Rights and permissions
Open Access This article is distributed nether the terms of the Artistic Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/four.0/), which permits unrestricted utilize, distribution, and reproduction in whatsoever medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/goose egg/1.0/) applies to the data made available in this commodity, unless otherwise stated.
Reprints and Permissions
About this commodity
Cite this article
Shen, Y., Abeynayake, R., Sun, X. et al. Feed nutritional value of brewers' spent grain residual resulting from protease aided protein removal. J Fauna Sci Biotechnol ten, 78 (2019). https://doi.org/10.1186/s40104-019-0382-1
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s40104-019-0382-1
Keywords
- Batch civilization
- Brewers' spent grain
- Chemical composition
- Fermentation
- Protease hydrolysis
Source: https://jasbsci.biomedcentral.com/articles/10.1186/s40104-019-0382-1
0 Response to "Whole Grain Brewing Beef Cattle Journal of Animal Science"
Post a Comment